Generics
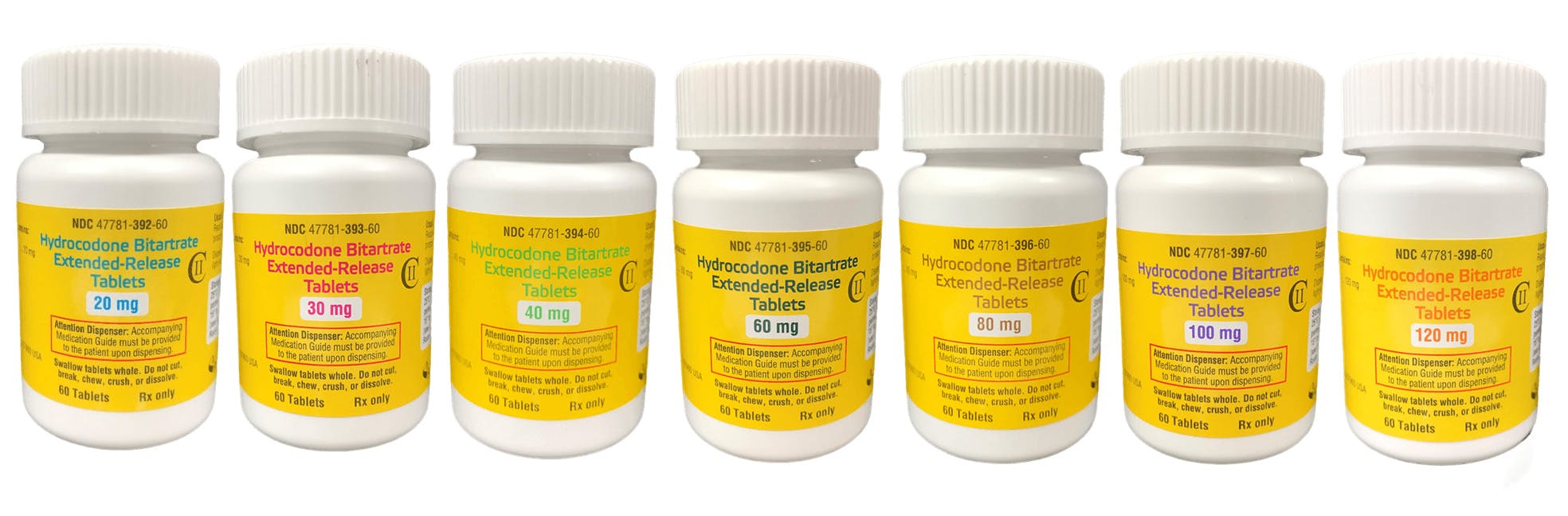
Hydrocodone Bitartrate Extended-Release Tablets C-ll
This product contains a BOXED WARNING. See full prescribing information for this product.
AB rated to: Hysingla® ER Extended-Release Oral Tablets*
| NDC# | 47781-392-60 |
|---|---|
| Strength | 20 mg |
| PKG size | 60 |
| GCN | 37539 |
| GCN SEQ# | 73176 |

| NDC# | 47781-393-60 |
|---|---|
| Strength | 30 mg |
| PKG size | 60 |
| GCN | 37541 |
| GCN SEQ# | 73177 |

| NDC# | 47781-394-60 |
|---|---|
| Strength | 40 mg |
| PKG size | 60 |
| GCN | 37543 |
| GCN SEQ# | 73179 |

| NDC# | 47781-395-60 |
|---|---|
| Strength | 60 mg |
| PKG size | 60 |
| GCN | 37544 |
| GCN SEQ# | 73180 |

| NDC# | 47781-396-60 |
|---|---|
| Strength | 80 mg |
| PKG size | 60 |
| GCN | 37545 |
| GCN SEQ# | 73181 |

| NDC# | 47781-397-60 |
|---|---|
| Strength | 100 mg |
| PKG size | 60 |
| GCN | 37546 |
| GCN SEQ# | 73182 |

| NDC# | 47781-398-60 |
|---|---|
| Strength | 120 mg |
| PKG size | 60 |
| GCN | 37547 |
| GCN SEQ# | 73183 |

*Trademarks (™) and registered trademarks (®) are property of their respective companies and not the property of Alvogen.